1. Executive Summary
About 50% of CO2 emitted in to atmosphere is from distributed sources such as transportation, aviation, etc. In net zero scenario, capturing CO2 directly from air (direct air capture, DAC) become very important. DAC has certain advantages as capture can be done at a place CO2 is required avoiding transportation and other costs. However, as CO2 concentration in air is very low (about 412ppm or 0.04%) DAC processes are every expensive as of now. The adoption of DAC is limited with the high cost of the relevant technology restricting its widespread deployment currently. There are two processes for DAC, viz., S-DAC (using solid adsorbent) and L-DAC (liquid absorbent). The existing processes are very capital and energy intensive resulting in high cost (USD200 – USD600 per ton of CO2 captured from air depending on technology, and other factors). It is estimated that about 95% of the cost of DAC is on account of capital and energy costs.
At CSIR-Indian Institute of Chemical Technology, Hyderabad, India, we have undertaken a project aimed at developing an alternate and commercially viable technology for DAC using phase change solvents. CSIR-IICT team screened several amines and found that few diamines on absorption of CO2 undergo phase change from liquid to solid state. Such amines also possess high CO2 sorption capacity- about 1.0 mole of CO2 / mole of amine compared to about 0.4-0.5 moles of CO2/mole of MEA (MEA – monoethanolamine is extensively used commercially for acid gas removal). IICT team has carried out studies on CO2 capture from air and from gas mixture with higher CO2 concentration on two phase change amines (aliphatic and alkyl-aromatic mono and diamines). Process parameters such as CO2 sorption capacity, regeneration temperature, and effect of solvent have been studied at laboratory scale in batch process. The lab studies are very promising as the sorption capacity of phase change amines studied is more than 1 mole CO2/mole of amine and the temperature required for regeneration is in the range of 70-80oC. This process is much simpler and much less energy intensive compared to L-DAC processes presently being employed at commercial scale. Proof of concept has been established and lab scale studies have been completed on couple of phase change amines on both absorption and desorption of CO2 and the reuse of spent amine reaching TRL-3. A continuous DAC process CO2 capture at bench scale is designed and facility with 1-2 kg per day CO2 capture from air is being established. There are no commercial process available using phase change amines for CO2 capture. Major focus of the ongoing work is on process optimization in terms higher CO2 capture efficiency, CO2 purity, and cost reduction with a target of USD100 per ton of CO2 captured. We are also working on the development of novel amines systems through molecular modelling and experimental studies.
The process being developed can also be used for other applications such as CO2 capture from flue gases from power plants, refinery off gases or from cement industry. As CO2 concentration in such streams is much higher than in air, the proposed process would work out to be substantially cheaper than the existing conventional amine process.
2. Current Status
Direct air capture (DAC) technologies extract CO2 directly from the atmosphere. Direct air capture plays an important role in net zero pathways. Capturing CO2 directly from the air provides a way to balance the emissions that are difficult to avoid, including from long-distance transport and heavy industry, as well as offering a solution for legacy emissions. CO2 captured from air can also be used as a climate-neutral feedstock for a range of products that require a carbon source. Another advantage is the possibility of locating DAC plants close to suitable storage or utilization sites, eliminating the need for long-distance CO2 transport.
In the IEA Net Zero Emissions by 2050 Scenario, direct air capture technologies are projected to be capture more than 85 Mt of CO2 in 2030 and around 980 MtCO2 in 2050, requiring a large and accelerated scale-up from almost 0.01 MtCO2 today. Currently there are 27 direct air capture facilities operating in Canada, Europe and the United States. Another 130 DAC facilities are likely to be in the pipeline and it is estimated that a cumulative capacity of 75 MtCO2/year will be installed which will still be less than 0.5% of global emission s per year. Recently, the first large-scale direct air capture plant of 500,000 tCO2/year (can be increased to 1 MtCO2/year) is being set-up in the Permian Basin, United States which is reported to become operational by the year 2024.
2.1 Existing Processes for DAC
Today there are two technology approaches for DAC viz., Solid and Liquid systems.
2.1.2 Liquid DAC (L-DAC) is based on two closed chemical loops. The first loop takes place in a unit called the contactor, which brings atmospheric air into contact with an aqueous basic solution (such as sodium hydroxide or potassium hydroxide) capturing CO2. The second loop Ca(OH)2 solution is mixed with Na2CO3 or K2CO3 forming CaCO3 which precipitates. CaCO2 precipitate is filtered, dewatered and subjected to calcination at high temperature (above 900oC) in a furnace/kiln to release the captured CO2. Both alkali and CaO are recycled in the process. Water top-up would be required depending on local weather conditions. For instance, around 4.7 tons of water per ton of captured CO2 would be required for this plant configuration at ambient conditions of 64% relative humidity and 20°C. Carbon Engineering is a major player in this technology DAC technology.
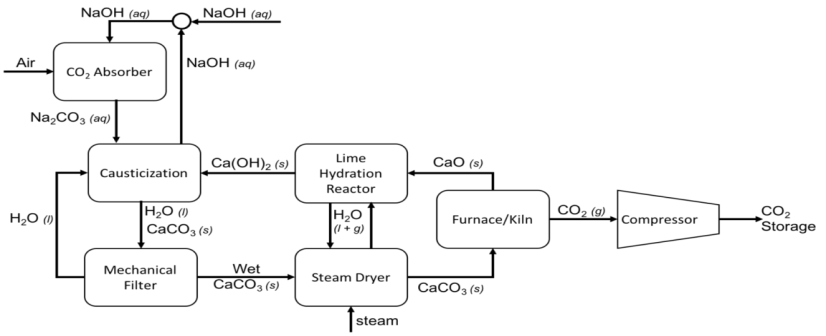
Fig 1. Schematic diagram of L-DAC process of Carbon Engineering.
2.1.1 Solid DAC (S-DAC) is based on solid adsorbents (e.g., amine-functionalized materials to enhance CO2 adsorption capacity) operating through an adsorption/desorption cyclic process. While the adsorption takes place at ambient temperature and pressure, desorption happens through a temperature–vacuum swing process, where CO2 is released at low pressure and medium temperature (80-100°C). A single adsorption/desorption unit can have a capture capacity of several tens of ton of CO2 per year (e.g. 50 t CO2/year) and can be used to extract water from the atmosphere where local conditions allow (early prototypes were able to remove around 1 ton of water per ton of CO2). An S-DAC plant is designed to be modular and can include as many units as needed. For instance, the largest operating S-DAC plant currently captures 4 000 ton of CO2 a year. As CO2 concentration in air is only 400ppm, for most of the adsorbents, adsorption capacity is very low. As both temperature and vacuum are required for regeneration, the process is energy intensive. As adsorption capacity is very low and cycle times are high for temperature swing adsorption process, the adsorbent productivity is very low. Climeworks is one of the major player in this technology.
3. Major DAC plants being set-up worldwide
Several major Direct Air Capture (DAC) of CO2 plants are coming up around the world, driven by the need to mitigate climate change by reducing atmospheric CO2 levels. Here are some of the notable ones are listed below:
3.1 Climeworks – Orca Plant, Iceland
- Location: Hellisheidi, Iceland
- Capacity: Initially captures 4,000 tons of CO2 per year
- Technology: Uses solid sorbent filters and geothermal energy for a sustainable process
- Significance: One of the largest operational DAC plants in the world, designed to be scalable to meet increasing demand (NIST) (CSIRO).
3.2 Carbon Engineering – Squamish, Canada
- Location: Squamish, British Columbia, Canada
- Capacity: Planned to capture up to 1 million tons of CO2 per year
- Technology: Uses an aqueous solution to capture CO2 from the air, which is then concentrated and purified
- Significance: Set to be one of the largest DAC facilities globally, aimed at producing synthetic fuels and other products (NIST).
3.3 Global Thermostat – Colorado, USA
- Location: Colorado, USA
- Capacity: Commercial plant designed to capture 1 million tons of CO2 per year
- Technology: Utilizes amine-based sorbents to capture CO2 from the air
- Significance: Focuses on integrating with industrial processes and utilizing captured CO2 for various applications (NIST) (World Economic Forum).
3..4 Project Bison – Wyoming, USA
- Location: Wyoming, USA
- Capacity: Aims to capture 5 million tons of CO2 per year by 2030
- Technology: Combines advanced DAC technology with geological storage
- Significance: Expected to be the largest DAC project in North America, contributing significantly to carbon sequestration goals (NIST).
3.5 Oxy Low Carbon Ventures and Carbon Engineering – Permian Basin, USA
- Location: Permian Basin, Texas, USA
- Capacity: Initial capture capacity of 1 million tons of CO2 per year, with plans for expansion
- Technology: Similar to Carbon Engineering’s Squamish plant, focusing on large-scale deployment
- Significance: Part of Occidental Petroleum’s strategy to use captured CO2 for enhanced oil recovery and eventual sequestration (NIST).
3.6 CSIRO’s ACOHA and CarbonAssist – Australia
- Location: Various locations in Australia
- Capacity: Pilot plants capturing up to 100 tons of CO2 per year, with plans for larger units
- Technology: ACOHA uses liquid amine-based capture technology; CarbonAssist uses a hybrid solid/liquid sorbent system
- Significance: Demonstrates a range of DAC technologies and their potential for scaling and integration with other industrial processes (CSIRO).
4. Efficiency and Energy Requirements
The efficiency and energy requirements of DAC are influenced by several factors. Some of the important factors are listed below.
- Sorbent Capacity: The ability of the sorbent to absorb CO2 at very low partial pressure.
- Air Flow Rate: The volume of air processed per unit time.
- Regeneration Energy: The energy required to release CO2 from the sorbent.
- Sorption and desorption cycle time: The cycle time would depend on the sorbent heating and cooling time required and have impact of sorbent productivity.
DAC is energy-intensive, with estimates ranging from 1.4 to 2.5 GJ (gigajoules) per ton of CO2 captured. Advances in materials science and process engineering are crucial to improving the efficiency and reducing the energy consumption of DAC systems.
5. Cost Analysis
The cost of DAC is currently high but expected to decrease with technological advancements and economies of scale. Key cost components include:
- Capital Costs: Infrastructure and equipment.
- Operational Costs: Energy, maintenance, and sorbent replacement.
Estimates for the current cost of DAC range from $200 to $600 per ton of CO2 captured. DOE target for DAC is less than $100 per ton of CO2 captured. Cost reductions can be achieved by:
- Improving Sorbent Performance: Enhancing the capacity, selectivity and regeneration temperature of sorbents.
- Optimizing Processes: Improving system design and reducing overall energy consumption.
- Scaling Up: Increasing the scale of DAC operations to benefit from economies of scale.
CSIR-IICT has been working on the development of liquid amine which undergoes phase change on CO2 absorption forming carbamic acid making it easy to separate the solid and regenerate at very low temperature (around 70-80oC). Such amines are reported to have high CO2 capture efficiency and also possess long term stability meeting all the requirements of a solvent for CO2 capture. Further, as the spent amine becomes solid, only the reacted amine needs to be heated and not the entire solution for regeneration. This would result in for in substantial reduction in overall energy requirement.
6. Strategy of CSIR-IICT
The proposed strategy is to use highly efficient low temperature regenerable phase change material and a suitable process for CO2 capture from air resulting in low energy and low cost.
6.1 Work done at IICT
In the initial CO2 capturing study, CO2 absorption was evaluated using simple batch process at room temperature (∼303 K). A number of sorbents have been screened and few suitable phase change sorbents have been identified. Sorbent regeneration has been studied at different temperatures and the captured CO2 could be completely desorbed at 70-80 Deg C. The reusability of sorbent under CO2 adsorption-desorption cycles showed >99% efficiency without degradation for 100 h under direct air capture conditions.
Generally, 10-30% of sorbent is mixed with a suitable solvent for CO2 capture. It is important to identify suitable solvent which helps in faster phase change. Optimum solvent has been identified by screening a number of solvents.
6.2 Process development
Based on experimental study done on the phase change amines for DAC at batch mode and at laboratory scale, a continuous process is designed for DAC. (Figure 2).
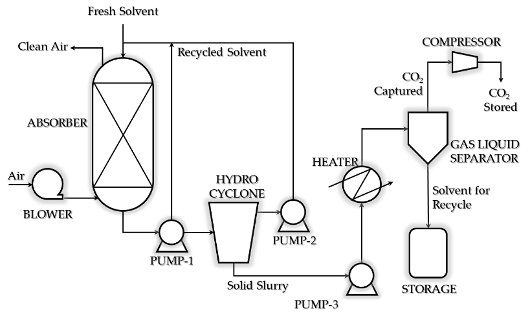
Fig. 2. Process schematic for Direct Air CO2 Capture through phase change absorption process
The proposed continuous unit has an absorber, hydrocyclone for separating over 90% of solvent which would be recycled. The reduced solid slurry would be pumped through line heater to gas liquid separator. CO2 gas separated would be compressed and stored. The solvent would be recycled.
6.3 Development of new solvents with higher CO2 capture efficiency
Aliphatic amines tend to be better sorbents in this case due to their higher basicity. H-bonding can also play a significant role in kinetics. Usually, faster kinetics is observed when two amine groups are closer. For example, cyclohexane-1,2-diamine showed faster carbamic acid formation than cyclohexane-1,3-diamine and cyclohexane-1,4-diamine, which could probably be due to the formation of H-bonding between carbamic acid and an adjacent amine group. Here faster kinetics is important to reduce the cycle time of the adsorption-desorption process. Furthermore, faster CO2-desorption kinetics at a relatively lower temperature is also necessary to form a perfect system. The new sorbent systems identified through molecular modelling would be synthesised in the lab characterized. The synthesised sorbents would be evaluated for their CO2 sorption efficiency, rate of adsorption and desorption temperature.
As solvent plays an important role, effect solvent would also be studied. Based on our preliminary studies, we propose that alkanes (e.g., heptane, octane, etc.) seems to be good solvents due to their non-toxic nature and miscibility with amines. Water would be our ultimate choice as a solvent but in some cases, the solubility can be very less when the molecular weight of amines increases.
7. Aims & Objectives
The major objective of the proposal is to develop a cost effective DAC process employing phase change amines. The sub objectives include:
- To develop and demonstrate a continuous DAC process using phase change amines with 1-1-2kg CO2 capture per day (1.4 to 2.8 million litres of air per day processing).
- To develop novel phase change sorbents based on molecular modelling studies.
- To generate process engineering data and design a large DAC plant (ton scale) for setting up in an industry in the next phase.
8. Expected Outcome
- Cost effective technology for CO2 capture by DAC using phase change sorbent.
- Efficient sorbent materials.
9. Target beneficiaries
- Hydrocar on Industry
- Power Industry
- Cement Industry
- Chemicals and pharma industry and
- Steel industry.
References
- Kikkawa, S.; Amamoto, K.; Fujiki, Y.; Hirayama, J.; Kato, G.; Miura, H.; Shishido, T.; Yamazoe S. Direct Air Capture of CO2 Using a Liquid Amine−Solid Carbamic Acid Phase-Separation System Using Diamines Bearing an Aminocyclohexyl Group. ACS Environ. Au 2022, 2, 354−362.
- Cai, H.; Zhang, X.; Lei, L.; Xiao, C. Direct CO2 Capture from Air via Crystallization with a Trichelating Iminoguanidine Ligand. ACS Omega 2020, 5, 20428−20437.
- National Academies of Sciences, Engineering, and Medicine; Negative Emissions Technologies and Reliable Sequestration: A Research Agenda. The National Academies Press. 2019.
- Fasihi, M., Efimova, O., & Breyer, C.; Techno-economic assessment of CO2 direct air capture plants. Journal of Cleaner Production, 2019, 224, 957-980.
- Keith, D. W., Holmes, G., St. Angelo, D., & Heidel, K.; A Process for Capturing CO2 from the Atmosphere. Joule, 2018, 2(8), 1573-1594.
- Seipp, C. A.; Williams, N. J.; Kidder, M. K.; Custelcean, R. CO2 Capture from Ambient Air by Crystallization with a Guanidine Sorbent. Angew. Chem., Int. Ed. Engl. 2017, 56, 1042−1045.
- Cuéllar-Franca, R. M.; Azapagic, A. Carbon Capture, Storage and Utilisation Technologies: A Critical Analysis and Comparison of Their Life Cycle Environmental Impacts. J. CO2 Util. 2015, 9, 82−102.
- Dijkstra, Z. J.; Doornbos, A. R.; Weyten, H.; Ernsting, J. M.; Elsevier, C. J.; Keurentjes, J. T. F. Formation of Carbamic Acid in Organic Solvents and in Supercritical Carbon Dioxide. J. Supercrit. Fluids 2007, 41, 109−114.
- https://www.csiro.au/en/news/All/Articles/2023/June/Direct-air-capture
- https://www.nist.gov/news-events/news/2024/04/nist-develops-new-testing-system-carbon-capture-fight-against-global.
- https://www.weforum.org/agenda/2023/10/accelerating-direct-air-capture-technology-global-warming/

Leave a reply to Carbon Capture – a Brief Review – Dr Nettem V Choudary Cancel reply